June 26, 2024 – Polyelemental materials exhibit intriguing properties across various domains, including catalysis, structural materials, and optoelectronic devices. Understanding mixing behaviors such as alloying and phase separation is important for correlating the structure and properties of these materials. Specifically, scaling down polyelemental materials to the nanoscale can significantly alter their physical and chemical characteristics. Now, researchers from the University of California, Berkeley, led by Peidong Yang and Kristin A. Persson, have systematically investigated the mixing behavior of the typically immiscible Au and Rh elements in nanoparticles ranging from 1 to 4 nm in size. They published their study in a recent issue of Nature Nanotechnology (https://doi.org/10.1038/s41565-024-01626-0).
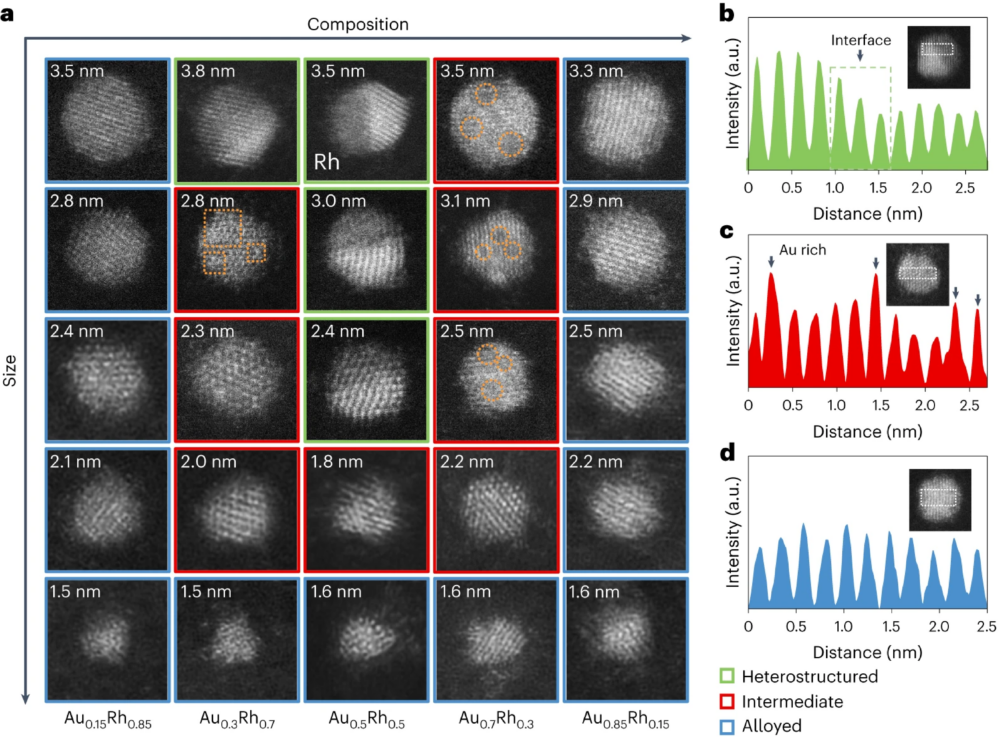
The synthesis process involved using polymer micelles with metal salt precursors as nanoreactors, depositing them onto microscopy grids and thermal annealing under Ar/H2 to synthesize Au–Rh nanoparticles of desired size (1 nm to 4 nm) and composition (Au85% to Au15%, denoting Au atomic percentage). Characterizations revealed that in nanoparticles larger than 4 nm, Au and Rh undergo phase separation into two distinct domains.
Peng-Cheng Chen, one of the co-first authors of the study, says, “Previously, I have developed methods for creating polyelemental nanoparticle libraries, demonstrating the synthesis of nanoparticles with various elements of specific size and composition. Given our capability to access numerous unprecedented nanoparticles, understanding their thermodynamic behavior becomes crucial.”
A two-dimensional (2D) design of experiments was conducted, with the x-axis representing AuRh nanoparticle composition and the y-axis denoting nanoparticle size. The researchers discovered that the mixing behavior of Au and Rh is highly dependent on both particle composition and size. When the particle composition ranges from 30% to 70% Au, the particles generally transition from heterostructured to intermediate and alloyed as the particle size decreases from 4 nm to 1 nm. In contrast, nanoparticles composed of 15% Au or 85% Au exhibited no size-dependent transition; for these compositions, Au and Rh can be alloyed across the entire 1–4-nm range.
Caitlin McCandler, another co-first author of the study, says, “The surface environment plays a crucial role in determining the phase stability of Au–Rh at the nanoscale. To address this challenge, we developed a thermodynamic model that incorporates surface passivation and other bulk properties to predict the phases of Au–Rh nanoparticles.”
Using density functional theory, the researchers determined the surface energy of Au and Rh, their interfacial energy, and their enthalpy of mixing. These parameters were integrated into thermodynamic models to calculate the total energy of Au–Rh nanoparticles and determine their thermodynamically stable configurations. The research team observed that smaller particle sizes accentuate surface effects, overriding the positive mixing enthalpy and promoting alloying. An equimolar elemental ratio maximizes the repulsion energy between Au–Rh, facilitating phase separation. Additionally, the model considers the potential adsorption of organic species, deviating from vacuum conditions. This study underscores the limited applicability of bulk phase diagrams, where Au and Rh only mix above 1000℃, to nanoparticle systems.
Younan Xia from the Georgia Institute of Technology, who was not involved in the study, says, “The findings of this paper will significantly expand the number of metals that can be combined to generate alloy materials with novel properties and applications. Improvements could focus on controlling the exact composition of the alloy and ensuring uniform composition across each particle, including the surface and subsurface regions.”
Original title: Alloying and phase separation explored in Au–Rh nanoparticles
Author: Nabojit Kar
Link: MRS Bulletin