Understanding the nature of structural evolution of Cu nanocatalysts during CO2 electroreduction is a long-standing question in catalysis science. Now, operando electron microscopy techniques correlated with X-ray spectroscopy and first-principles modelling demonstrate that the reaction products themselves drive the structural evolution in Cu nanocubes.
The selective catalytic reduction of CO2, also called the CO2 reduction reaction (CO2RR), is a key catalytic reaction, both from a fundamental materials chemistry perspective, and because of its potential for generating synthetic fuels from sequestered CO2 (ref. 1). Owing to the high bond-dissociation energy of the carbonyl bonds in CO2 (724–757 kJ mol−1, among the highest for small molecules)2, the reaction either proceeds extremely slowly without an effective catalyst or requires elevated temperatures and pressures. Of the types of catalyst that have been studied, copper is one of the most promising candidates because of its relative abundance compared to platinum-group metals and its ability to catalyse the formation of hydrocarbons and alcohols from CO2 with high selectivity3.
Given that catalysis is such a surface-driven phenomenon, the use of nanocrystals, which have large surface-area-to-volume ratios as catalysts, is a natural choice. Additionally, tuning the shape and size of nanocrystals allows researchers to access properties that may not be present in the bulk material4, providing further ways to modify catalyst behaviour. However, this added tuneability comes at a cost, because these structures are much less stable compared to their bulk counterparts and undergo reconstruction under reaction conditions. Observing such structural changes is non-trivial because these changes happen quite rapidly and happen at small length and time scales. Experimentally, this is enormously challenging because it is necessary to see the structures and chemistry of these nanoparticles evolving in real time at a resolution high enough to understand the origin of the structural evolution and then correlate the structural transformations with changes in the catalytic activity.
Now, writing in Nature Catalysis, Yang, Mavrikakis and colleagues5 have answered this question using a combination of operando X-ray microscopy, high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and 4D STEM7 (a STEM technique where the 2D diffraction pattern is collected for every scan position in a 2D scan grid, resulting in a 4D dataset) to observe nanoparticle structural evolution in real time, and also demonstrated the origin of these structural transformations using surface-enhanced Raman spectroscopy (SERS) and density functional theory (DFT). They demonstrate that shape-controlled copper nanocubes do not remain static during the CO2RR reaction and that even an initially faceted, oxide-passivated nanocube will undergo continuous reconstruction under CO2 electrolysis conditions.
An interesting and unexpected finding from the study is the influence of nanocatalyst size on reconstruction pathways during catalysis. Upon applying a mild reducing potential, the approximately 2-nm-thick Cu2O surface shell of the 55-nm-per-side cubes was reduced to metallic copper, forming an amorphous, low-density spongy coating around the crystalline core. At the CO2RR operating potential this spongy Cu shell nucleated into small Cu clusters or seeds. Subsequently, the underlying Cu core itself began to break up until the entire cube was converted into a collection of polycrystalline Cu nanograins. In other words, each large 55-nm Cu nanocube eventually reconstructed into a porous aggregate of Cu grains under CO2RR, as seen in Fig. 1a.
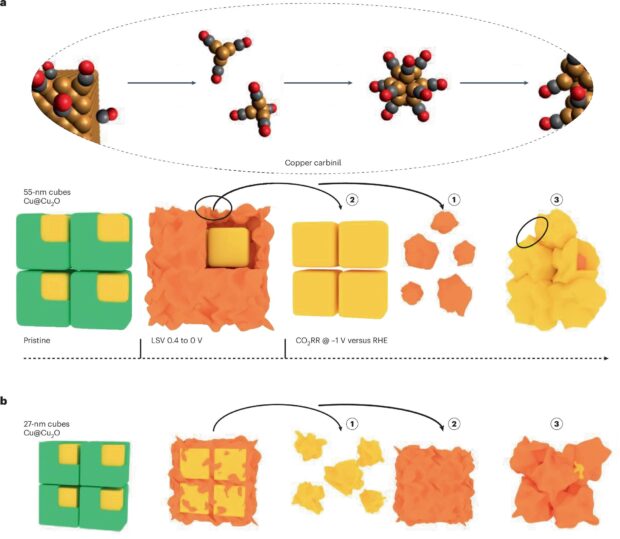
In contrast, the smaller 27-nm-per-side cubes followed a different pathway because their copper cores started transforming earlier and faster than the shell, as seen in Fig. 1b. For these smaller nanocubes, the core fragmented first to produce an irregular cluster, and then the remaining partial Cu2O shell reduced and migrated till it reached a polycrystalline nanograin state very similar to that of the larger copper cubes. Whether the surface oxide or the metallic core reacts first is dependent on size — an insight which was only made possible by observing this behaviour in real time.
These results could be collected because the researchers’ use of liquid-cell STEM (LC-STEM) allowed them to capture the nanocube structural evolution in real time while an electrochemical potential was applied. This operando HAADF-STEM imaging directly visualized the formation of the spongy Cu shell and the emergence of Cu nanograins from the original cube through a stepwise evolution: the nucleation of tiny clusters, the growth and merging of those clusters, and eventual fragmentation of the Cu core. Additionally, the use of dose-controlled LC-STEM demonstrated the different structural evolution pathways of the 27-nm and the 55-nm copper nanocubes. When combined with operando 4D-STEM6 along with unsupervised machine learning, it became possible for the researchers to extract the structural signatures of these transformations and observe the ratio of amorphous to crystalline phases by using fluctuation electron microscopy8.
Next, the study addresses what causes this different behaviour to happen. Through in situ SERS and DFT calculations, the researchers found that CO actively interacts with the Cu surface to mobilize Cu atoms. Specifically, CO was seen to bind strongly at low-coordination sites (edges/defects) on Cu and could even extract single Cu atoms from the lattice. The ejected Cu atoms, stabilized by CO, migrated on or near the electrode surface and formed new Cu clusters. The evidence for this mechanism comes from time-resolved SERS spectra. The adsorbed CO band at around 1,900 cm–1 that comes from CO bound to isolated Cu sites decreased over time, while a broad band in the 2,000–2,100 cm–1 range (which was characteristic of CO on Cu nanoclusters or surfaces) grew9. DFT simulations corroborated this CO-driven atom migration because at high CO coverage the calculations showed that a surface Cu atom can detach while bound to a CO molecule, forming a neutral Cu–CO complex that is thermodynamically favourable. The ejection barrier for a Cu atom drops with increasing local CO coverage, consistent with CO facilitating the removal of Cu from the step edges.
The overall evolution of the copper nanocubes is thus a complex process and each technique applied in this study gives us a piece of the puzzle. LC-STEM and 4D-STEM provide the real-space and diffraction-space structural information, respectively, while XAS and SERS supply the chemical signatures of this transformation and help to explain the mechanism behind it.
The observation of extensive structural transformation in the Cu nanocrystals raises the question of whether this is a bad thing for catalysis. Perhaps surprisingly, the answer is that it is not. In the studied system, the copper nanocubes evolved into a steady-state architecture that maintained high ethylene selectivity over extended operation. This indicates that rather than fighting against surface changes, structural evolution can be harnessed to our advantage. For instance, by pre-forming a thin oxide coating, as was done here, we could direct the formation of a beneficial porous morphology under CO2RR. Furthermore, the size-dependent behaviour of the nanocube structural evolution implies that tuning the initial particle size could be a strategy to influence the rate of reaching the stable state and the nature of the resulting final active surface.
Read the full article here.
Original title: Operando imaging reveals nanocatalyst reconstruction
Author: Debangshu Mukerjee